By Athayde Tonhasca
As the story goes, during a tour of a government farm, American First Lady Grace Coolidge was being shown around by a farmer when she saw a cockerel and a hen romantically engaged. She asked her guide how often the cockerel would mate, to which he responded: ‘dozens of times a day.’ Good-humouredly, Mrs Coolidge retorted: ‘tell that to the President’. The farmer dutifully did so, and President Calvin Coolidge asked: ‘same hen every time?’, to which the farmer replied: ‘No, Mr President, a different hen every time.’ And the president: ‘tell that to Mrs Coolidge.’
Psychology Professor Frank A. Beach (1911-1988) saw this improbable anecdote as an ideal model to name a widespread phenomenon among animals: the Coolidge Effect, which is the enhanced sexual interest of males whenever a new female is accessible, regardless of the availability of previous sexual partners – a behaviour rarely reported for females. This shocking manifestation of male chauvinism has been offered a biological explanation.
The term ‘gonochorism’ makes us scramble for the dictionary, even though one of the first things we learned from our Birds and Bees lessons is that our species is gonochoric (or dioecious), that is, it has two sexes: the male sex produces or is geared up to produce gametes (reproductive cells) called sperm, while the female sex is equipped to produce gametes known as ova or egg cells. The lesson’s climax was the revelation that some types of frolicking could result in the fusion of these two types of gametes to produce babies.

Later in life, when we took biology courses, we were told that many plants and some animals are hermaphrodites (they produce male and female gametes), while other organisms don’t need sex to reproduce. But the overwhelmingly majority of animals, and all mammals and birds, are sexually binary: they either produce male gametes or female gametes – leaving aside the rare cases of individuals that don’t fit in either category. And, from humans to asparagus, that is, for virtually all multicellular organisms, the female gametes are larger – often much larger – than the male gametes; that’s to say they are anisogamous: the two types differ in size and shape. And anisogamy has much to do with the Coolidge Effect.
Because sperm are relatively small, energetically cheap gametes, males can afford to churn out and distribute lots of them. By mating with as many females as possible, males increase their chances of passing on their genes. If a male gamete ends up in an unsuitable female, it’s not a big deal: there are plenty more fish in the sea. It doesn’t work like that for females. They put a lot of energy into their eggs, which are gigantic when compared to sperm. So, a female can only make a few of them in her lifetime. Adding gestation and time spent nurturing their young, females have a much lower reproductive capacity. As they invest a great deal more in producing an embryo than males, they need to choose their mates well to maximize their chances of success; if their Romeos are weak and unfit, females may have wasted all their reproductive potential. For females, it’s a matter of quality, not quantity.
These biological particularities are strong incentives to polygyny, the mating system where a male has multiple sexual partners while the female mates with one or a few males. Polygyny is the most common mating strategy for vertebrates; about 90% of mammal species are polygynous. These males are, like the Coolidges’ rooster, always ready for a new romantic adventure.
Angus John Bateman (1919–1996), a botanist who worked with fruit flies, found one important consequence of the Coolidge Effect. For most polygynous species, a small number of males monopolize the females and prevent other males from mating. That is, some males are highly successful in reproducing, while many more have no success at all. Things are more predicable for females: most of them will mate – the few successful males will make sure of that. The upshot is that males’ reproductive success is more variable than females’.

Enter evolutionary biologist Robert Rivers and computer scientist Dan Willard (1948-2023) to thicken the plot by proposing that differences in reproductive success can bias the production of male and female offspring. Trivers and Willard argued, reasonably, that sons and daughters of females in good condition (that is, well-fed, healthy, and not pressured by competitors) would also be in good condition, whereas sons and daughters of females in poor condition (malnourished or debilitated by parasites or competitors) would also be in bad condition. But, when the reproductive success of one sex – males, in the case of polygynous species – is more variable than the other, diverging strategies emerge. It pays for strong, healthy females to have many sons, who mate frequently and produce lots of grandchildren for their mother. Daughters on the other hand are a less promising investment because, despite being strong like mum, they are restricted by low reproductive rates. But if the mother is in poor condition, having daughters would be a better deal because despite being feeble like mum, those who survive to adulthood are likely to produce some offspring. Feeble sons on the other hand may never breed, as they would be no match for males in good condition (Trivers & Willard, 1973). In other words, when things get bad, it’s better to have more daughters than sons. This risk-spreading strategy is a form of biological bet-hedging to maximize fitness and applies beyond mammal polygyny. If females’ reproductive success is more variable, we should expect more sons than daughters when the going gets rough.
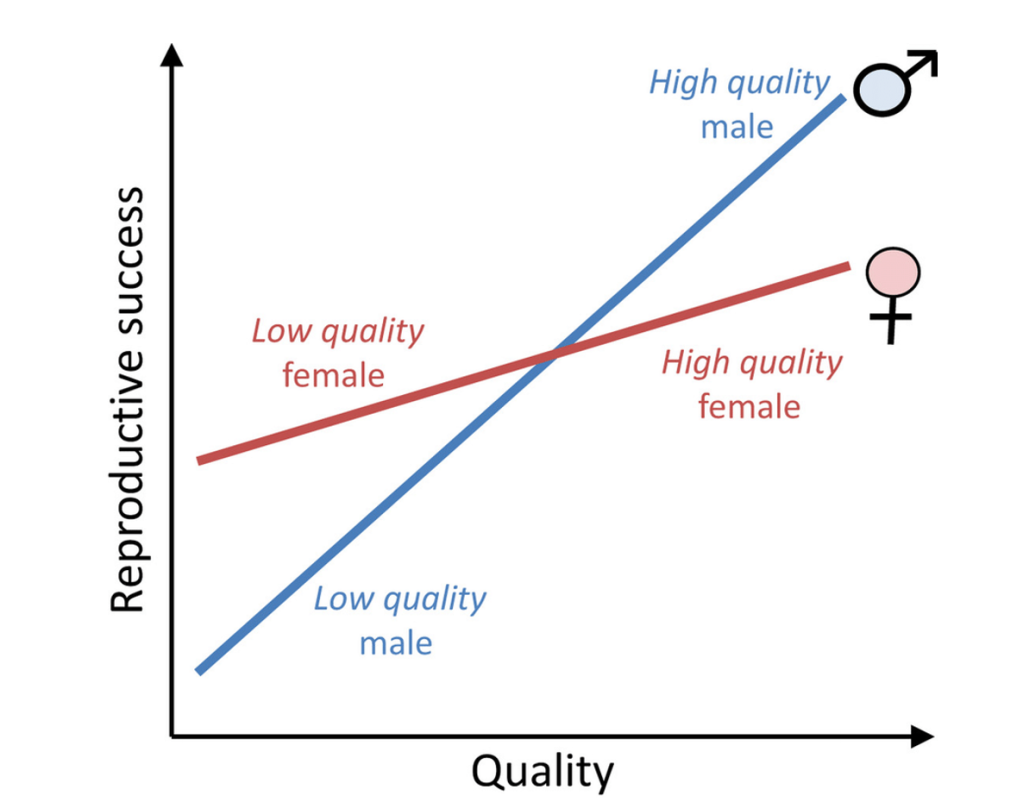
The Trivers–Willard hypothesis provides an explanation for a common occurrence among animals: sex ratios going astray. In theory, a species should produce about the same number of sons and daughters (1:1 ratio) to maintain long term stability. This is known as the Fisher’s principle – although it would be fairer to call it the ‘Cobb’s principle’ after the solicitor and amateur biologist John Cobb (1866-1920), who first proposed it (Gardner, 2023).
The Trivers–Willard hypothesis has had an enormous influence in evolutionary biology. Its predictions have been supported by studies with a range of species, although its universality has been debated and questioned. Nonetheless, the hypothesis has encouraged much theoretical and empirical research about sex allocation. This body of work has revealed that variation of reproductive success between sexes is not the only driver of sex ratio skewness. Food, mothers’ age, litter size, population density, the weather, or some other environmental or physiological factor may induce females to adjust the sex ratio of their offspring to maximise fitness.
It turns out that food availability is an important inducer of sex ratio fine-tuning for one group of animals of enormous ecological end economic importance: cavity-nesting solitary bees. Most of the 20,000 or so known species of bee build their nests in the ground, but about 30% of them took another path regarding housing. They occupy or expand naturally occurring cavities such as crevices under or between stones, cracks in a wall, holes in dead wood, hollow stems and tree bark, transforming them into cosy, safe environments in which to raise their young.
Like all solitary bees, cavity-nesting species are on the wing for a small portion of their lives, sometimes weeks. After mating, each female spends her short adult life tirelessly victualing her nest with pollen and nectar to provide for her brood. It’s a race against time and over hurdles such as bad weather, competitors, flower scarcity, pests and parasites. Reproductive success depends on the amount of food available for the young, and here their sex can be the decisive factor. Female bees – like most insects – are in general bigger than males, so they need more food. As these big eaters could be a survival risk, some tinkering may be in order.


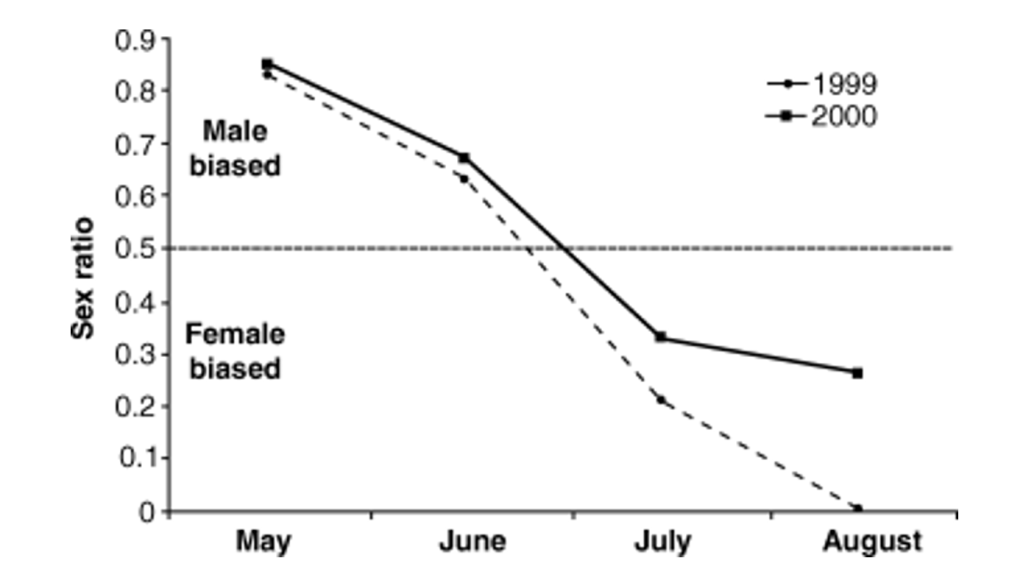
The orchard mason bee or blue orchard bee (Osmia lignaria), a cavity-nesting species from North America, is a valued pollinator of several fruit trees. During the early nesting season, when pollen and nectar are most abundant and mum is in top shape, her offspring comprise mostly females. As the season progresses, flowers become scarce, so she has to work harder to provision her nest. Now the sex ratio tilts towards the smaller males, who have better chances of survival because they need less food (Torchio & Tepedino, 1980).
The scenario is similar for the related red mason bee, a Eurasian species, but here parasites play a part. As the nesting season advances, females become less efficient and take more time to gather food, creating opportunities for nest-invading parasites. Females deal with the problem by reducing the amount of food stored, with a corresponding shift in the sex ratio towards the less demanding sons (Seidelmann, 2006). In the case of the Australian endemic banksia bee (Hylaeus alcyoneus), the growing food scarcity causes the reduction of the brood’s body mass and a shift in their sex ratios. But contrary to the prevailing pattern found in bees, male banksia bees are significantly larger than females. So unsurprisingly, the energetically cheaper daughters became more abundant late in the season (Paini & Bailey, 2002). Other cavity-nesting bees have also shown declines in foraging efficiency as the season progresses, and these changes have been linked to reduced size of their offspring and shifts in their sex ratios.

The facultative, condition-dependent shift of sex ratios is a remarkable survival tool. The power to quickly tilt the offspring’s sexual balance could make the difference for a species’ success. In the non-nonsense, unforgiving great outdoors, where long-term existence hangs on the ability to adapt to changes, boys and girls are not always equally valued: these are the times when a Sophie’s choice of sorts is necessary.







