By Athayde Tonhasca
In an apple orchard somewhere in the American state of Pennsylvania, an adult Japanese horn-faced bee (Osmia cornifrons) has just emerged from its nest and makes its way into the big wide world. The apple grower has high hopes for that bee; in fact, he bought many of them when they were still inside their cocoons. The Japanese horn-faced bee was introduced from Japan in the 1970s, and since then it has been widely used in the Eastern United States to improve the pollination of apples and other fruit trees such as peaches, pears and cherries.

In their natural habitats, the Japanese horn-faced bee and similar species such as the red mason bee (O. bicornis) nest inside natural cavities such as hollowed reeds, tree holes and cracks in stones. Females use a range of materials, especially mud and pebbles, to build individual nest cells in which they lay an egg. When bees are done, they seal off the nest entrance with mud – so they are known as mason bees. Fruit growers offer bees nesting alternatives such as drilled blocks of wood or bunches of cardboard tubes tightly packed together.

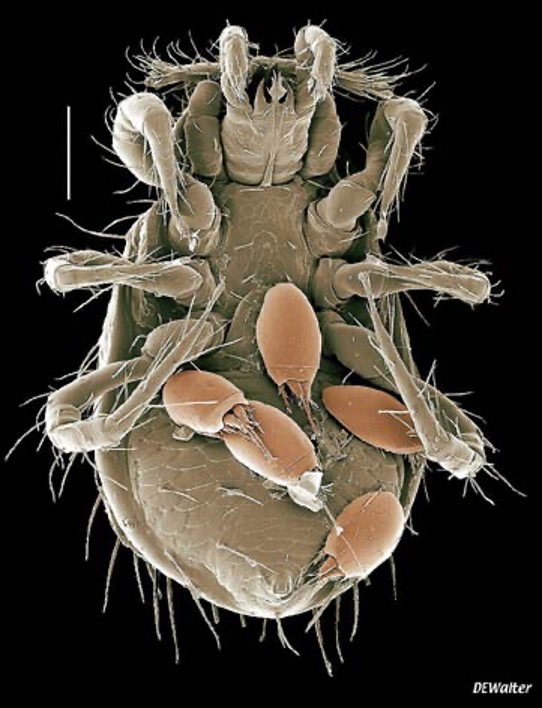
The future seemed promising for that Japanese horn-faced bee in Pennsylvania. But opportunists were on standby, ready to pounce when an unsuspecting bee leaves its nest. In the blink of an eye, a gang of hypopi (singular hypopus) jumps on the bee, holding on for dear life as their ride flies away.
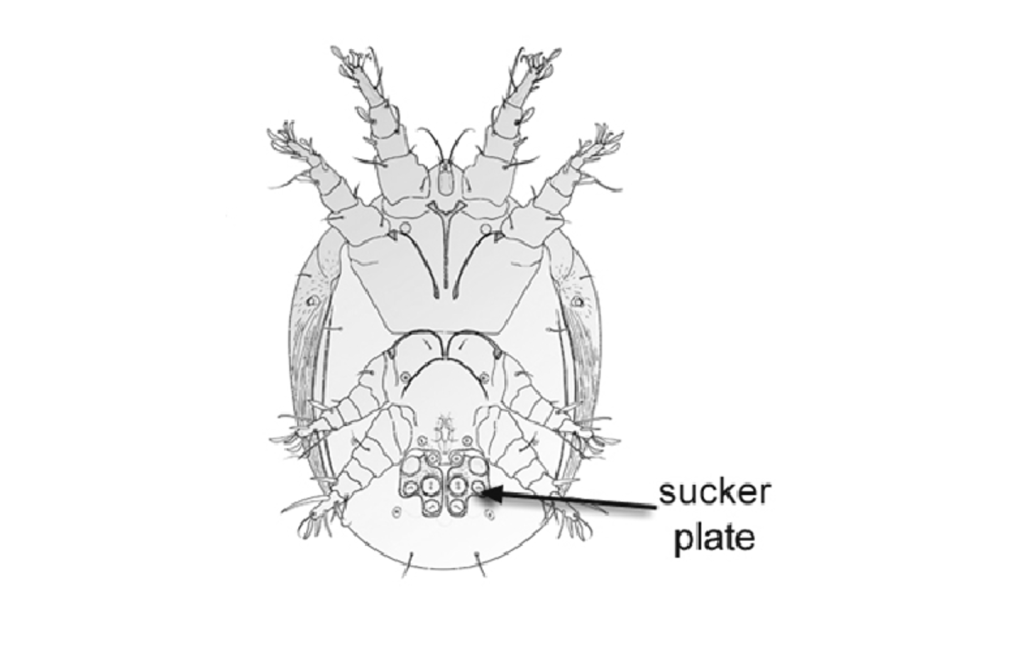
Hypopi, also known as hypopodes, are a special nymphal stage found in some mites. In this case, the hairy-footed pollen mite (Chaetodactylus krombeini). Hypopi have no head or mouthparts, but are armed with special structures for hanging on; either powerful claws or a sucker plate to glue themselves to their host. These adaptations greatly facilitate phoresis, which is when an organism attaches itself to another for the purpose of transportation. Phoresis is typically found in small and poorly mobile organisms such as nematodes and mites. But curiously, the hypopus stage is usually facultative for mites; it occurs only when conditions deteriorate (food scarcity, overcrowding, dry climate, etc.), so that skedaddling increases the likelihood of survival.
The departing bee has no chance of avoiding the lurking hitchhikers who react instantaneously to the slightest touch to their dorsal setae (bristles) or to air movement caused by a passing body. And the feats of some of these mites defy credulity; the tiny Histiostoma laboratorium (formally known as H. genetica), a scourge of vinegar fly (Drosophila melanogaster) laboratory colonies, lurches into the air to grab fruit flies flying above them (Hall, 1959. J. Kansas Entomological Society 32: 45-46). Some species that have hummingbirds as hosts rush to the birds’ nostrils at a rate of 12 body-lengths per second, which is a speed similar to a cheetah’s (Colwell, 1985)
After being mobbed by hypopi, the bee carries on with its life. If it’s a female, she will mate and start a nest of her own. When her brood cells are ready, her unwanted companions come out of their lethargic state, jump off and resume their development, maturing and reproducing very quickly, all the while feeding on the pollen and nectar gathered by the bee. When their numbers reach certain levels, they may feed on the bee’s eggs and larvae (details are sketchy). In a few months the mites may reach thousands and overrun the brood cell, leaving space for nothing else.
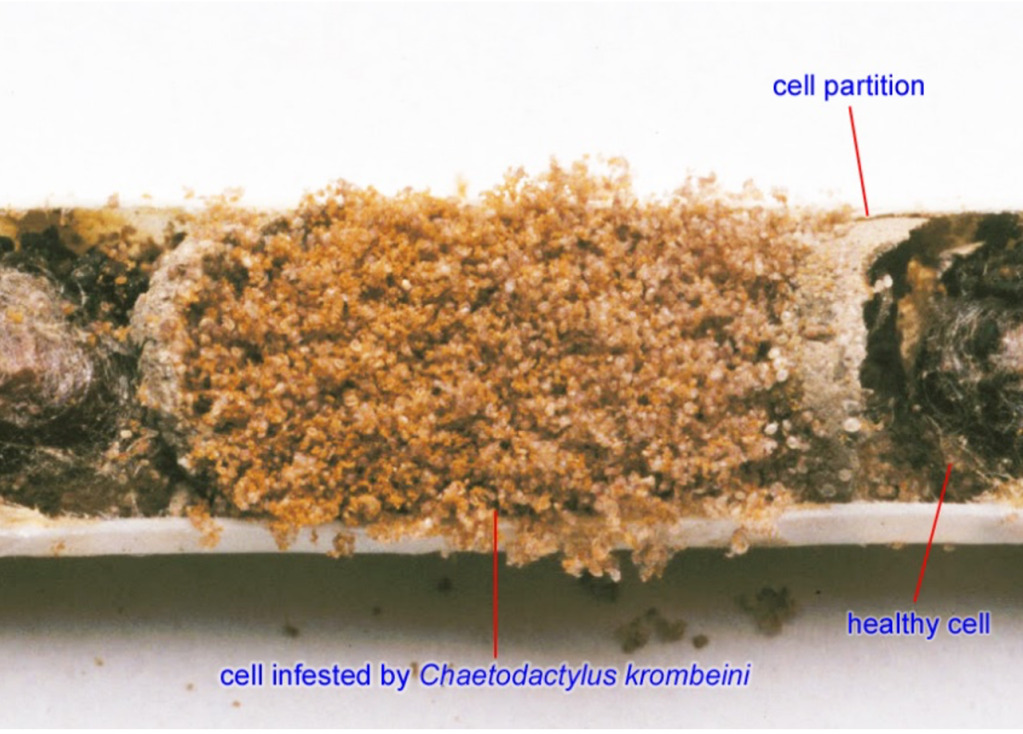
Such massive numbers of kleptoparasites (organisms that steal food from another one) spell serious trouble for Japanese horn-faced bees; their eggs and larvae die or develop poorly for lack of food or direct attack from mites. Some adult bees may not even have a chance to start a new family; they may be so burdened by mites that they cannot fly. They fall to the ground and become easy pickings for ants and other predators.

Several mason bee species are susceptible to the hairy-footed pollen mite, but managed Japanese horn-faced bees have been hit particularly hard, with losses reaching up to 50% of the population. It’s not difficult to understand why. The same way slum housing conditions make people more vulnerable to all sorts of diseases, jam-packed nests increase the chances of mites passing from one bee to another. And the hairy-footed pollen mite does not even depend on phoresis: adults can walk from one nest to another nearby, getting inside through holes in the sealing mud made by parasitic wasps. To make the situation worse, this mite can turn into a dormant stage that survives several years inside an empty nest, rousing back to activity as soon as new tenants arrive.
The effects of the hairy-footed pollen mite on the Japanese horn-faced bee are a reminder of the unintended consequences of well-intentioned actions. Bee houses or bee ‘hotels’ have been promoted as enhancers of wild bee populations, but there’s no indication of such effects. They do however increase the risk of pathogens and parasites: not only mites, but a range of fungi, parasitic flies and wasps bedevil mason bees (Groulx & Forrest, 2017).

American fruit growers do their best to keep mites under control by replacing the nesting tubes yearly, sterilising wood blocks, or removing and storing bee cocoons during the winter. If you have a bee house but don’t have the resources, time or inclination to do the same, you should follow Colin Purrington‘s advice: buy a garden gnome instead.





